Are you still waiting for your vaccination? WCFIA Fellow Mario Jimenez of Gavi, the Vaccine Alliance, reports on the multifaceted efforts to supply the world with the vaccines necessary to end the COVID-19 pandemic.
By Mario Jimenez
While Americans are preoccupied with the arrival of the Pfizer and Moderna vaccines in their states and struggle to get appointments, major players are at work around the world creating more than seventy different vaccines. It’s true that Western countries have locked up most Pfizer and Moderna orders; timely availability of vaccines remains uncertain; and Russian and Chinese vaccines struggle to gain trust around the world. But the good news is that an unprecedented global collaboration is taking place among leading pharmaceutical companies, private and country donors, and public health officials to provide vaccines equitably to countries with the fewest resources.
The COVID-19 pandemic is one of the most significant exogenous shocks to human society in the past century. Unexpected, at least in its timing, COVID-19 has paralyzed the global economy, imposed substantial changes in our lifestyles, and resulted, so far, in more than 115 million documented infections, 2.5 million deaths, and tens of trillions of dollars in damages. The financial, political, and societal effects that have been caused by this event make it an inflection point in modern history that will require thorough analyses in the years to come.
In response to the pandemic, scientists, the pharmaceutical industry, and global public health entities have embraced the previously unimaginable goal of developing and releasing a safe and effective vaccine within one year. Fifteen months after the first human infections were reported by officials in Wuhan City, China in December 2019, twelve vaccines have been approved for full or emergency use around the world, seventy-six vaccine candidates are in clinical trials on humans, and over 150 are in preclinical development.
These achievements are mainly the result of important clinical and manufacturing innovations that are commensurate with the magnitude of the crisis that the world is facing. What is new, however, and might serve as a model for future health emergencies, is that the involved actors do not just accelerate the regular process of vaccine development, but work in parallel across a multitude of processes— development,registration, funding, manufacturing set-up, production of vaccine drug substance, securing commercial sales, and preparation for distribution—all at the risk that the vaccine may not even work.
Western government action, which in the past has often been criticized as sedate and bureaucratic in times of crises, has now launched new forms of support such as underwriting costs of research, indemnification, and “same-side-of-the-table” collaboration with regulators in real time to address outstanding concerns rather than post hoc review. Additionally, funding agencies from North America, Europe, and the Asia-Pacific region have unlocked significant resources for research on COVID-19, fostering collaboration across the public and private sectors and information sharing to address major technical challenges and barriers.
Safe and effective vaccines in a laboratory environment are only the first step
The pandemic as such and its above-described impact on communities, trade, and health can only be deemed under control when billions of vaccine doses will have been produced and made available to everyone. Regardless of ethical considerations, it is a matter of efficiency, and it is in the interest of the containment of the virus and preventing deaths that distribution begins with frontline health workers and other high-risk groups. Furthermore, the long-term success of the undertaking—and thus the protection of each one of us—can be secured only if the ability to pay for the vaccine is not a factor which accelerates or decelerates distribution. It goes without saying that this titanic mission requires entities that had no need to rely on each other in the past to now work closely together.
Twelve months after the World Health Organization (WHO) declared the pandemic, our success remains partial. In sum, the wealthiest countries in North America and Europe have locked up orders for most of the vaccine supply—enough to vaccinate two or three times their populations—leaving poor countries, and thus the vast majority of the world’s population, struggling to secure their share and destined to wait until 2022 or later before supplies are widely available.
While developing countries, particularly in Africa, have been able to avoid the high mortality rates seen in Western countries, the economic and societal disruptions from the pandemic in developing countries have been devastating. Strict lockdowns, which were ordered to preserve the often-fragile healthcare sectors, have caused other pandemics: hunger, disease, and illiteracy. Recent research shows that the greatest impact of the COVID-19 pandemic may not be on those whom the virus infects directly, but on those most affected by the collapse of the economy, the monopolization of the healthcare system, and the neglect of education (day laborers; children missing routine immunization vaccines, dying of malnutrition, and missing school; girls forced into marriage and abuse).
While only few of such negative effects of the pandemic could have been prevented, the key for shortening worldwide suffering, namely the reactivation of the economies and the opening of societies, can be achieved only by a speedy and universal rollout of the available vaccines.
A coordination mechanism toward equitable and fair access to COVID-19 vaccines
The COVID-19 Vaccines Global Access Facility, or Covax Facility, is an initiative led by Gavi, the Vaccine Alliance, along with the Coalition for Epidemic Preparedness Innovations, and WHO. It was established by bringing together state and private donors, governments, technical partners, and vaccine manufacturers to ensure eventual COVID-19 vaccines reach those in greatest need, equitably.
This initiative started under the assumption that no single vaccine is guaranteed to succeed or has enough capacity to supply the global volume required—an assumption which has proved to be true. Therefore, a diversified portfolio of vaccine providers has been engaged in the Covax Facility to spread risk and create capacity to scale together. By pooling financial and scientific resources, the participating economies can insure themselves against the failure of an individual vaccine candidate and secure access to those vaccines which are proven successful in a cost-effective, targeted way.
This is how it works: developed economies are asked to self-finance their vaccines through the Covax Facility, thus contributing to a pool procurement mechanism and the scale-up of manufacturing capacity of the most promising vaccines, while low-income eligible countries benefit from donor resources for the procurement of vaccines for up to 20 percent of their populations, and from access to further vaccines at the negotiated prices.
This global effort involving thousands of highly motivated healthcare sector specialists has set itself an immodest objective: end the acute phase of the pandemic by the end of 2021, deliver at least two billion vaccine doses through the management of the largest portfolio of vaccine candidates globally, and at its core, guarantee fair and equitable access to all participants.
As of February 2021, 1.84 billion doses have already been secured through signed agreements and around 630 million doses are under active negotiation for delivery throughout this year, with the first vaccines to become available in Ghana, Angola, and the Democratic Republic of the Congo. Confirmed vaccine manufacturers participating in the Covax Facility include some of the most important actors in the field: Serum Institute of India, AstraZeneca, Pfizer/BioNTech, Johnson & Johnson, and Sanofi/GSK.
COVID-19 vaccine landscape
When vaccine candidates enter human clinical trials, they first go through phase 1 trials. This step is set to primarily test the vaccine’s safety, determine dosages, and identify any potential side effects in a small number of people. Phase 2 trials further explore safety and start to investigate efficacy on larger groups. The final stage before approval, phase 3 trials, which few vaccines ever make it to, are much larger, involving thousands or tens of thousands of people, to confirm and assess the effectiveness of the vaccine and test whether there are any rare side effects that become manifest only in large groups. Even after vaccines are approved for use, long-term clinical studies designed to better understand the risks and potential benefits over time are conducted. These clinical trials are often called “open-label studies,” or phase 4 trials.
According to WHO, as of March 2, 2021, there are seventy-six vaccine candidates in clinical development: twenty-five are in phase 1 undergoing safety tests in healthy individuals; thirty are being tested in broader groups of people in phase 2; seventeen are in large international trials to test their impact on COVID-19 in phase 3; and four in phase 4. Twelve vaccines are currently approved and licensed for general or emergency use.
There are four main types of COVID-19 vaccines: whole virus, protein subunit, nucleic acid (RNA and DNA), and viral vector vaccines:
The whole virus type follows the conventional approach to trigger an immune response through the use of either a weakened form of the virus (live attenuated vaccines, like the current oral Polio vaccine) that can still replicate without causing illness, or an inactivated form which uses viruses whose genetic material has been destroyed so they cannot replicate but can still trigger an immune response. While both versions use well-established technology, live attenuated ones have a risk of actually causing the disease that they are supposed to prevent, namely in people with weak immune systems. They also often require careful cold storage, making their use more challenging in low-resource countries. Inactivated virus vaccines can be given to people with compromised immune systems but might also need cold storage. Multiple factors in research and development make one or the other version more likely to succeed, thus qualifying it as a suitable vaccine despite the here-mentioned risks and storage challenges. Leading COVID-19 vaccines using this technology include Sinopharm, Sinovac, and Bharat Biotech.
Protein subunit vaccines use only parts of the virus to trigger an immune response. Doing so minimizes the risk of side effects, but it also means the immune response may be weaker. Protein subunit vaccines often require adjuvants to help boost the immune response. An example of such vaccines in the current setting is the Novavax vaccine.
Nucleic acid vaccines use genetic material from the virus—either RNA or DNA—to provide healthy human cells with the instructions to make the spike protein themselves. Once this genetic material gets into our bodies, it uses our own cells' protein factories to make the antigen that will trigger an immune response. The advantage of such vaccines is that since the antigen is produced inside our own cells and in large quantities, the immune reaction is known to be strong. A downside, however, is that RNA vaccines need to be kept at ultra-cold temperatures, -94F or lower, which could prove challenging for countries that do not have specialized cold storage equipment, particularly low- and middle-income countries. Pfizer-BioNTech and Moderna vaccines are examples of this technology.
Lastly, viral vector vaccines use a similar technology by giving cells genetic instructions to produce their own antigens. However, they differ from nucleic acid vaccines in that they use a harmless virus, different from the one the vaccine is targeting, to deliver these instructions. One type of virus that has often been used as a vector is the adenovirus, which causes the common cold. As with nucleic acid vaccines, our own cellular machinery is “hijacked” to produce the antigen from those instructions in order to trigger an immune response. The Astrazeneca-Oxford vaccine is a leading COVID-19 vaccine using this technology.
Unlike what usual media coverage suggests, the big players of the manufacturing business in the current crises are not always the most visible ones: the Serum Institute of India, the world’s largest vaccine manufacturer, is playing a critical role enabling supply of vaccines to developing countries such as Ivory Coast, Nigeria, and Senegal through their partnership with Astrazeneca and Novavax.
The Astrazeneca vaccine was granted Emergency Use Listing by WHO on February 15, 2021, giving the green light for these vaccines to be rolled out through Covax. Considering its efficacy of over 80 percent, refrigerator stability (meaning that it can be easily transported anywhere in the world), and its competitive price compared to other leading vaccines, it makes an important contribution to the effort of worldwide vaccine coverage. In addition to vaccine production in India, AstraZeneca has manufacturing networks in South Korea, China, Argentina, Mexico, and Europe—but each network will need a separate regulatory approval to ensure equivalency with the originator product.
It is expected that several other vaccines for which the Covax Facility already has agreements, such as the single-dose Johnson & Johnson/Janssen vaccine, Novavax from multiple producers, and the Sanofi/GSK candidate will become available in the second half of 2021, enabling further access to low-income countries.
Challenges ahead
Despite the noteworthy progress in vaccine development, many uncertainties affecting the supply of COVID-19 vaccines remain. Supply timing largely depends on quality control procedures and timelines, including review of individual batches. Manufacturing productivity is influenced by multiple factors, which in turn will influence volume and timing of supply. The economic lockdown also has a negative impact on the production of devices which are needed to fight the very cause of the lockdown. And finally, timing of delivery depends on local regulatory approval and overall country readiness and capacity to manage logistics and successfully deploy the vaccines. In some countries, vaccines are available but the healthcare sector to administer them is understaffed. Other countries have their health workers waiting and ready to act but no vaccine to inject.
It is common that logistics, supply chain issues, civil unrest, conflict, and natural disasters undermine successful vaccine delivery in developing countries. Under the current pressure on many weak national health systems—not only caused by the pandemic, but also due to the associated disruptions and the increased risks of outbreaks of other diseases such as measles, polio, and yellow fever—a successful rollout of the COVID-19 vaccine is a daunting mission.
Technical partners such as WHO and UNICEF, NGOs, and multiple immunization actors around the world continue to mobilize to support national and subnational efforts toward the preparation for COVID-19 vaccination. Countries continue to work on regulatory preparedness, planning, and coordination efforts, budgeting and funding, identification of target populations and delivery strategies, preparation of supply chains, management of health care wastage, vaccine safety monitoring, management of adverse events, and vaccine acceptance and uptake strategies.

Skeptical populations resistant to the coronavirus vaccine are part of the challenge. In Pakistan, the mistrust around vaccines has been fueled by anti-Western conspiracy theories spread across social media and pushed by extremist religious groups. Questions regarding vaccines affecting reproductive ability, using technology to track people’s movements, and killing people are common. Additionally, widespread mistrust in China’s product is also leading to refusal of the first vaccine introduced in the country. Understanding the perception of vaccines across population groups, including their fears and beliefs, is therefore crucial to the development of tailored solutions.
The recent emergence of rapidly spreading variants of the COVID-19 virus makes the fast and equitable rollout of vaccines of greater urgency. Two broad types of mutations have been identified: some that make the virus more infectious, and others that appear to make it capable of evading antibodies generated by vaccines. While scientists are trying to understand the spread of the new variants and how these may affect existing therapies, vaccines, and tests, antibodies are just one component of immune protection. Another component is T-cell immunity, which offers stronger protection against a serious progression of the illness. If the virus mutates, it does not have an effect on our T-cell immunity.
Given the increasing number of emergency use authorizations for COVID-19 vaccines by regulatory authorities, some countries such as Norway and the European Union countries have now secured sufficient doses to begin sharing a portion of them with other countries. Consequently, the Covax Facility is adapting its mission and also works with potential dose-sharing countries and vaccine manufacturers to include these doses in the Covax Facility and to facilitate their equitable global distribution. Such additional doses can accelerate the facility’s goal of ensuring participating countries coverage of up to 20 percent of their population as soon as possible in 2021—and can expand coverage beyond that in 2022.
The United States, as one of the Vaccine Alliance’s original six donor countries, has been a long-supporting contributor to the mission of ending preventable child deaths through vaccination. Most recently, in December 2020, it has approved an additional commitment of $4 billion of support to the Covax Facility, making it the largest contributor. Germany is the second largest contributor with a support of $1.1 billion.
The COVID-19 pandemic has set the world’s attention on the important role vaccines play in protecting lives, livelihoods, and economies, and on the critical role of international cooperation to overcome global challenges. Covax represents the largest global effort to enable rapid, fair, and equitable access to COVID-19 vaccines, and therefore aims to bring this pandemic to the swiftest possible end and restore peace and security across the globe.
—Mario Jimenez, Fellow, Weatherhead Scholars Program
Mario Jimenez is a Fellow in the Weatherhead Scholars Program at the Weatherhead Center for International Affairs. He is also a program manager at Gavi, the Vaccine Alliance. His research interests include global public health; health financing; health policy; vaccines; access to medicines; health care systems; and universal health coverage.
Captions
- Vaccine vial with syringe isolated over blue background. Credit: Shutterstock
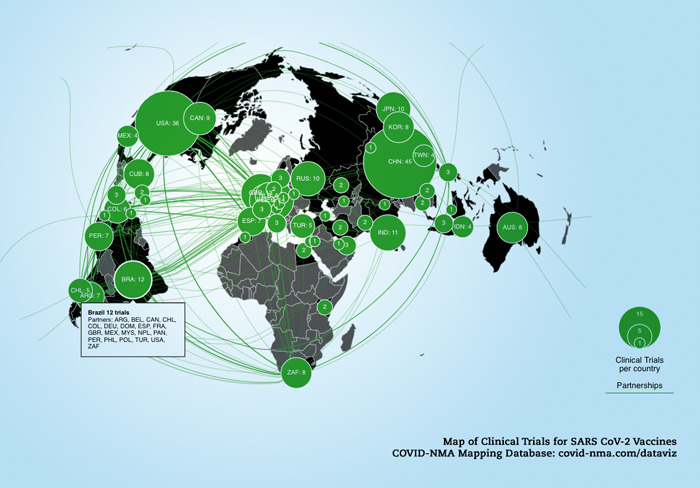
- Map of clinical trials for SARS CoV-2 vaccines. As of February 26, 2021 the Covid-19 - living NMA initiative collected 171 RCTs and 52 non-randomised studies of vaccines from the ICTRP. Credits | Data: COVID-19 - living NMA initiative. Visualizations: Romain Vuillemot - LIRIS, École Centrale de Lyon; Philippe Rivière - LIRIS, VisionsCarto; Pierre Ripoll - LIRIS, INSA Lyon; Julien Barnier - Centre Max Weber, CNRS. Retrieved from: https://covid-nma.com/vaccines/mapping/ (CC BY 4.0: color changes made)
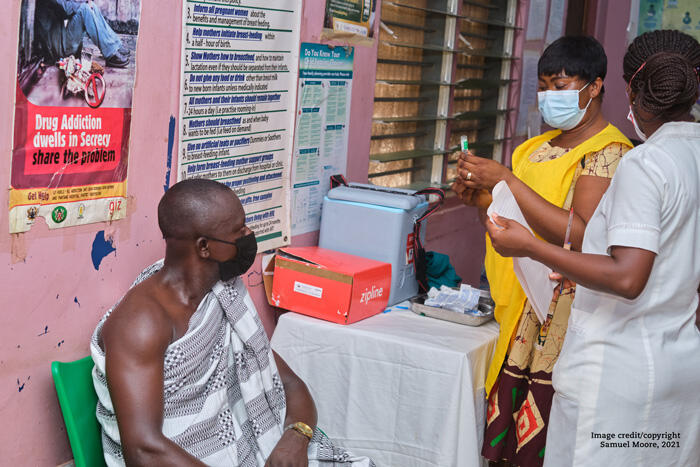
- On Tuesday, 2 March, a health worker at the Asuofua Health Center in Ghana's Ashanti region draws up a shot of a COVAX COVID-19 vaccine, delivered earlier the same day by a Zipline drone. Zipline, in partnership with UPS and the Government of Ghana, and supported by Gavi, is committed to helping Ghana achieve equitable access to COVID-19 vaccines. Part of an initial COVAX delivery of 600,000 doses, these first vaccinations targeted health workers on the front line and government officials. Credit: Samuel Moore / 2021
- There are four types of COVID-19 vaccines: here’s how they work. Credit: Gavi, the Vaccine Alliance, YouTube
